SAFETY FIRST: We're taking extra measures to ensure your children are safe in our school. More details
Sharadha, N., Kumari, H. K. and Reddy, R. J.* Asian J. Org. Chem., 2024, e202400211.

Kumari, H. K., Kumar, J. J., Sharadha, N., Krishna, G. R. and Reddy, R. J.*. ChemSusChem, 2024, e202400227.

Reddy, R. J.* Kumar, J. J. and Kumari, H. K. JOrg. Biomol. Chem. 2024, 22, 2492-2509..

Reddy, R. J.* Sharadha, N. and Krishna, G. R. J. Org. Chem. 2023, 88, 8889-8903..

Reddy, R. J.* Kumar, J. J. and Kumari, H. K. Org. Lett. 2023, 25, 2207–2212.

Reddy, R. J.* Kumari, H. K. and Krishna, G. R. J. Org. Chem. 2023, 88, 1635.

Reddy, R. J.* Kumar, J. J. and Krishna, G. R. Adv. Synth. Catal., 2022, 364, 4080.
R. J. Reddy,* Md. Waheed, T. Karthik, and A. Shankar, New J. Chem., 2018, 42, 980-987.
_edited-3.jpg)
R. J. Reddy,* Md. Waheed, T. Karthik, and A. Shankar, New J. Chem., 2018, 42, 980-987.

Reddy, R. J.* Sharadha, N., Kumari, A. H. Org. Biomol. Chem. 2022, 20, 4331-4337

15. Solvent-Dependent Mono- and Bis-Thiolation of (E)-beta-Iodovinyl Sulfones with Thiols for
Flexible Synthesis of 1,2-Thiosulfonylalkenes and 1,2-Dithioalkenes
Reddy, R. J.* Kumari, A. H., Sharadha, N. and Krishna, G. R., J. Org. Chem. 2022, 87, 3934−3951

14. Diethyl phosphite-mediated switchable synthesis of bis(imidazoheterocycles) derived disulfanes and sulfanes using imidazoheterocycles and octasulfur (Invited)
Reddy, R. J.* Shankar, A., Kumar, J. J. Sharadha, N., and Krishna, G. R., New. J. Chem., 2022, 46, 4784
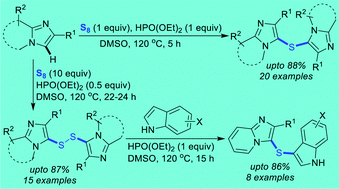
13. Interrupted CuAAC-Thiolation for the Construction of 1,2,3-Triazole-Fused Eight-Membered
Heterocycles from O-/N-Propargyl derived Benzyl Thiosulfonates with Organic Azides
Reddy, R. J.* Waheed, Md., Kumari, A. H., and Krishna, G. R., Adv. Synth. Catal. 2022, 364, 319-325.

12. Ni–Catalyzed Difunctionalization of Alkynyl Bromides with Thiosulfonates and N-Arylthio
Succinimides: A Convenient Synthesis of 1,2-Thiosulfonylethenes and 1,1-Dithioethenes
Kumari, A. H., Kumar, J. J., Krishna, G. R and Reddy, R. J.,*, Synthesis, 2021, 53, 2850–2864.

11. Recent Advances in the Synthesis and Applications of b-Keto Sulfones: New Prospects for the
Synthesis b-Keto Thiosulfones (Review article)
Reddy, R. J.,* Kumari, A. H. and Kumar, J. J., Org. Biomol. Chem. 2021, 19, 3087-3118.

10. Simple and Efficient Synthesis of Allyl Sulfones through Cs2CO3-mediated Radical Sulfonylation
of Morita-Baylis–Hillman Adducts with Thiosulfonates (Highlighted in the SynForm News)
Shankar, A., Waheed, Md. and Reddy, R. J.*, SynOpen, 2021, 5, 91–99 (Invited).

Reddy, R. J.* and Kumari, A. H., RSC Adv., 2021, 11, 9130–9221.


08. Phenylboronic acid-catalyzed tandem construction of S–S and C–S bonds: a new method for the
synthesis of benzyl disulfanylsulfone derivatives from S-benzyl thiosulfonates
Reddy, R. J.,* Waheed, Md. and Krishna, G. R., Org. Biomol. Chem. 2020, 18, 3243-3248.

07. Pd-Catalysed Annulation of beta-Iodovinyl Sulfones with 2-Halophenols: A General Route for the
Synthesis of 3-Sulfonyl Benzofuran Derivatives
Reddy, R. J.,* Kumar, J. J., Kumari, A. H. and Krishana, G. R. Adv. Synth. Catal., 2020, 362, 1317-

06. Efficient, Sequential One-Pot Approach for Diverse C3-Functionalized Imidazo[1,2-a]-pyridines Under Transition-Metal Free Conditions
Reddy, R. J.,* Shankar, A. and Kumari, A. H.; Asian J. Org. Chem., 2019, 8, 2269-2275.
05. Unprecedented Reactivity of beta-Iodovinyl Sulfones: An Efficient Synthesis of betoKeto Sulfones and beta-Keto Thiosulfones
Reddy, R. J.,* Kumar, J. J. and Kumari, A. H.; Eur. J. Org. Chem., 2019, 3771-3775.
Reddy, R. J.,* Kumari, A. H., Kumar, J. J. and Nanubolu, J. B.; Adv. Synth. Catal. 2019, 361, 1587-
03. A straightforward and convenient synthesis of functionalized allyl thiosulfonates and allyl
Reddy, R. J.,* Waheed, Md. and Kumar, J. J. RSC Adv., 2018, 8, 40446–40453.
02. Metal-free highly regioselective sulfonylation of NH-1,2,3-triazoles with sodium sulfinates and
Reddy, R. J.,* Shankar, A., Waheed, Md. and Nanubolu, J. B., Tetrahedron Lett., 2018, 59, 2014-2017.
cycloaddition-denitration process
R. J. Reddy,* Md. Waheed, T. Karthik, and A. Shankar, New J. Chem., 2018, 42, 980-987.
-------------------------------------------------------------------------------------------------------------------------------------------------------------------
Publications during the Postdoc (UK, Japan and Taiwan) and PhD (Univ. of Hyderabad)
18. Alkynyl thioethers in gold-catalysed annulations to form oxazoles
R. J. Reddy, M. P. Ball-Jones and P. W. Davies, Angew. Chem. Int. Ed., 2017, 56, 13310–13313
17. General Entry into o-,o’-Heteroatom-Linked N-(Hetero)aryl Imidazole Motifs by Gold-Catalysed
Formal [3+2]-Dipolar Cycloaddition
M. Garzon, E. M. Arcea, R. J. Reddy, and P. W. Davies, Adv. Synth. Catal., 2017, 359, 1837-
1843 (designated as VIP and Most accessed articles in 05/2017)
16. Efficient and Flexible Synthesis of Highly Functionalised 4-Aminooxazoles by a Gold-Catalysed Intermolecular Formal [3+2] Dipolar cycloaddition,
Gillie, A. D.,+ Reddy, R. J.,+ and Davies, P. W., Adv. Synth. Catal., 2016, 358, 226-239 (+equally
contributed; designated as VIP and most accessed articles)
15. Synthesis of 1-Phenethyltetrahydroisoquinoline Alkaloids (+)-Dysoxyline, (+)-Colchiethan-amine
and (+)-Colchiethine
Reddy R J, Kawai N and Uenishi J. J. Org. Chem., 2012, 77, 11101–11108.
14. Kinetic resolution of activated nitroallylic acetates with aldehydes and ketones via conjugate
addition-elimination SN2' process
Reddy R J, Lee P-H, Magar D R, Chen J-H and Chen K, Eur. J. Org. Chem., 2012, 353-36.
13. Highly Efficient Organocatalytic Kinetic Resolution of Activated Nitroallylic Acetates with
Aldehydes via Conjugate Addition-Elimination
Reddy R J and Chen K, Org. Lett., 2011, 13, 1458-1461.
12. An efficient Morita-Baylis-Hillman reaction for the synthesis of multifunctional 2-hydroxy-3-
nitrobut-3-enoate derivatives;
Kuan H-H,† Reddy R J† and Chen K, Tetrahedron, 2010, 66, 9875-9879 (†equal contribution).
11. Pyrrolidinyl-Camphor Derivatives as a New Class of Organocatalysts for Direct Asymmetric
Michael Addition of Aldehydes and Ketones to β-Nitroalkenes
Ting Y-F, Chang C, Reddy R J, Magar, D R and Chen K, Chem. Eur. J., 2010, 16, 7030-
7038 (Most accessed articles in 5/2010).
10. The Baylis-Hillman adducts as a valuable source for one-pot multistep synthesis: A facile
synthesis of 5-substituted-2-piperidones
Basavaiah D, Reddy R J and Lenin D V, Helv. Chim. Act., 2010, 93, 1180-1186.
09. Remarkable reaction Rate and excellent enantioselective direct a-amination of aldehydes with
azodicarboxylates catalyzed by pyrrolidinyl-camphor derived organocatalysts
Liu P-M, Chang C, Reddy R J, Ting Y-F, Kuan H-H and Chen K, Eur. J. Org. Chem., 2010, 42- 46 (Most accessed articles in 11/2009).
08. Novel Prolinamide-Camphor Containing Organocatalysts for Direct Asymmetric Michael
Addition of Unmodified Aldehydes to Nitroalkenes
Reddy R J, Kuan H-H, Chou T-Y and Chen K, Chem. Eur. J., 2009, 15, 9294-9298 (Most
accessed articles in 08/2009).
07. Pyrrolidine-Camphor Derivative as an Organocatalyst for Asymmetric Michael Additions a,a-
Disubstituted Aldehydes to beta-Nitroalkenes: Construction of Quaternary Carbon Bearing
Aldehydes under Solvent-Free Conditions
Chang C, Li S-H, Reddy R J and Chen K, Adv. Syn. Catal., 2009, 351, 1273-1278 (Most
accessed articles in 06/2009).
06. Highly diastereo- and enantioselective direct aldol reactions promoted by water-compatible
organocatalysts bearing a pyrrolidinyl-camphor structural scaffold
Tzeng Z-H, Chen H-Y, Reddy R J, Huang C-T and Chen K, Tetrahedron, 2009, 65, 2879–2888.
05. Simple and facile synthesis of tetralone-spiro-glutarimides and spiro-bisglutarimides from
Baylis-Hillman acetates
Basavaiah D and Reddy R J, Org. Biomol. Chem., 2008, 6, 1034-1039.
04. The Baylis-Hillman reaction: a novel source for attraction, opportunities, and challenges in
synthetic chemistry (Review Article)
Basavaiah D, Rao K V and Reddy R J, Chem. Soc. Rev. 2007, 36, 1581-1588.
03. Applications of Baylis-Hillman adducts: A simple, convenient, and one-pot synthesis of 3-
benzoylquinolines
Basavaiah D, Reddy R J and Rao J S, Tetrahedron Lett., 2006, 47, 73-77.
02. TiCl4 catalyzed tandem construction of C-C and C-O bonds: a simple and one-pot atom-
economical stereoselective synthesis of spiro- oxindoles
Basavaiah D, Rao J S, Reddy R J and Rao A J, Chem. Commun., 2005, 2621-2623.
01. Simple, facile and one-pot conversion of the Baylis-Hillman adducts into functionalized 1,2,3,4-
tetrahydroacridines and cyclopenta[b]quinolines
Basavaiah D, Rao J S and Reddy R J, J. Org. Chem., 2004, 69, 7379-7382.
--------------------------------------------------------------------------------------------------------------------------
02. Book Chapter: ‘Sustainable Catalysis’ Other 2-substituted pyrrolidines as asymmetric organocatalysts
Reddy R J and Chen K, Royal Society of Chemistry Publishers, 2015, Volume 2, Chapter 9, pp
200-235 (ISBN: 9781782620570).
01. Book monograph: Applications of Baylis-Hillman adducts: A novel strategies for the synthesis
of spiro and heterocyclic compounds
Reddy R J; LAMBERT Academic Publishing, 2-Dec-2012; ISBN-10:365921707; EAN: 9783659201707.


Laboratory of OrganoSulfur Chemistry
Dr Raju J Reddy Research Group
Department of Chemistry, University College of Science
Osmania University, Hyderabad, India
